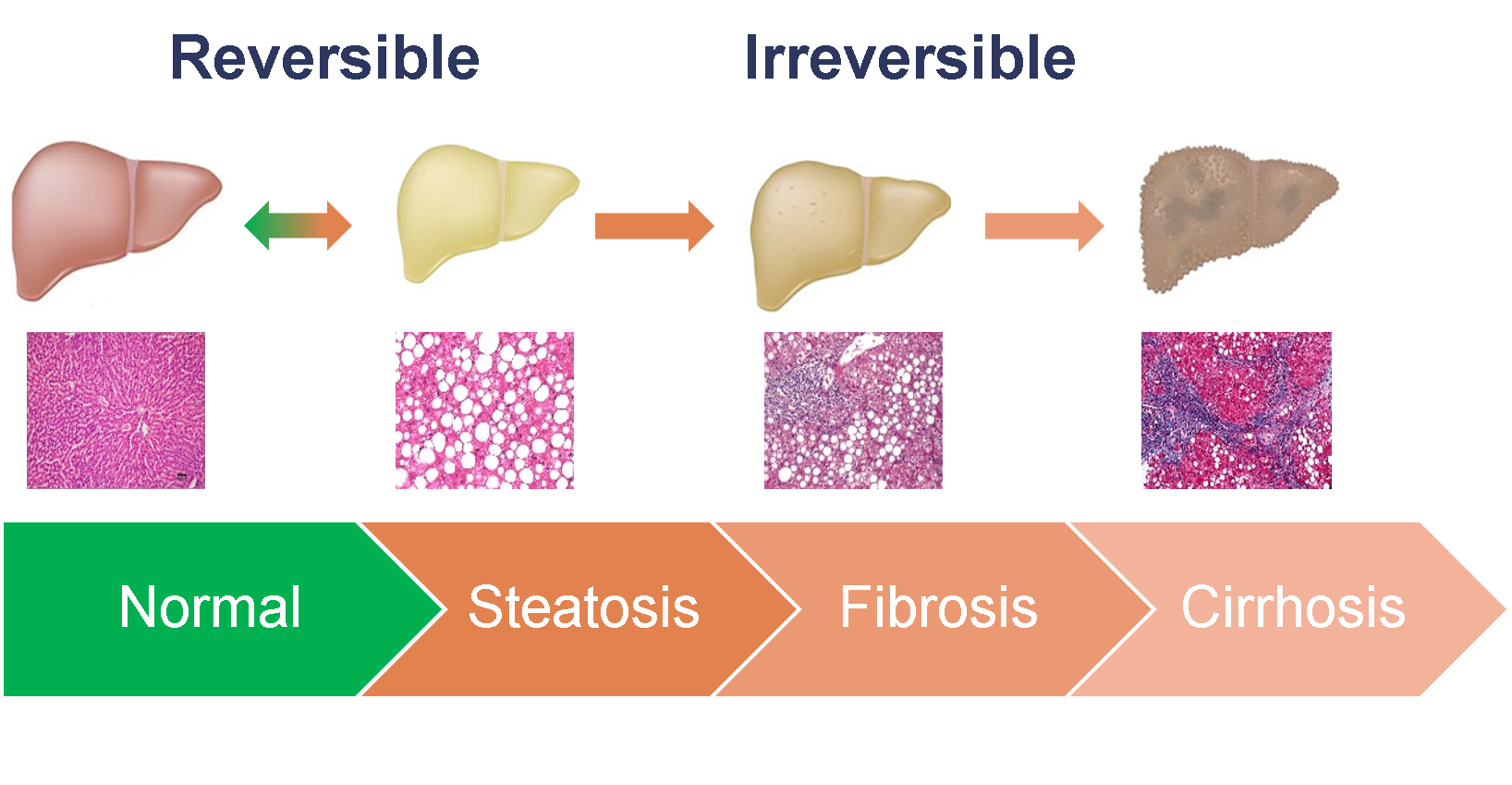
Liver Steatosis Detection
Non-alcoholic fatty liver disease (NAFLD) affects 1 in 4 persons, globally [1]. Steatosis (excessive accumulation of fat in hepatic cells) is the
first stage of NAFLD. This condition is reversible, but can progress into fibrosis and cirrhosis. Liver biopsy
is not feasible for routine screening due to its potential risk and prohibitive cost. Thus, there is a significant interest in developing reliable,
inexpensive and non-invasive biomarkers to detect and monitor the progression of NAFLD. Fat droplets in the fatty liver cause cellular ballooning,
which affects the ultrasonic scattering process, resulting in an increase in attenuation coefficient estimate (ACE). Based on this principle, ACE
can be utilized as a promising tool to detect and quantify hepatic steatosis.
 Unfortunately, ACE methods based on a sliding window approach suffer from the trade-off between image resolution and estimation precision and accuracy.
Larger windows improve accuracy and precision by reducing the spatial variation noise inherent in ultrasonic scattering, whereas smaller windows better
resolve the underlying structure. Additionally, variation in scatterer size and concentration inevitably creates inhomogeneity in tissue, which results
in a large error in ACE estimation.
To address these issues, We have developed a spatially weighted total variation regularization based reference phantom method (SWTV-ACE) for ACE estimation [1,2]. In this
method, the local regularization is modified as a function of envelope signal-to-noise-ratio deviation, an indicator of tissue inhomogeneity.
Unfortunately, ACE methods based on a sliding window approach suffer from the trade-off between image resolution and estimation precision and accuracy.
Larger windows improve accuracy and precision by reducing the spatial variation noise inherent in ultrasonic scattering, whereas smaller windows better
resolve the underlying structure. Additionally, variation in scatterer size and concentration inevitably creates inhomogeneity in tissue, which results
in a large error in ACE estimation.
To address these issues, We have developed a spatially weighted total variation regularization based reference phantom method (SWTV-ACE) for ACE estimation [1,2]. In this
method, the local regularization is modified as a function of envelope signal-to-noise-ratio deviation, an indicator of tissue inhomogeneity.
We show an example of ACE computation for in vivo liver to highlight the advantage of SWTV-ACE over traditional methods. Here an inhomogeneity indicated by
high envelope SNR deviation causes significant variation of
ACE (including negative ACE values) computed using the reference phantom method and the uniform TV regularization method. However, SWTV-ACE method yields
positive ACE values with low estimation variance.
Additionally, we investigated the effect of axial window size on the estimation variation of ACE for
a homogeneous region-of-interest (max(ΔSNRe)<20%) and an inhomogeneous region-of-interest in liver (mean(ΔSNRe)>20%). For the homogeneous case, both
uniform TV regularization and SWTV-ACE methods maintain similar standard deviation for different window sizes, whereas the reference phantom method exhibits
the trade-off where estimation precision improves with increasing window size. For the inhomogeneous case, however, SWTV-ACE method outperforms both the
reference phantom method and the uniform TV regularization by maintaining similar standard deviation for different window sizes (Fig. 1(b)). Therefore, SWTV-ACE
effectively improves the quality of ACE computation by reducing the variability in the estimates irrespective of window size.
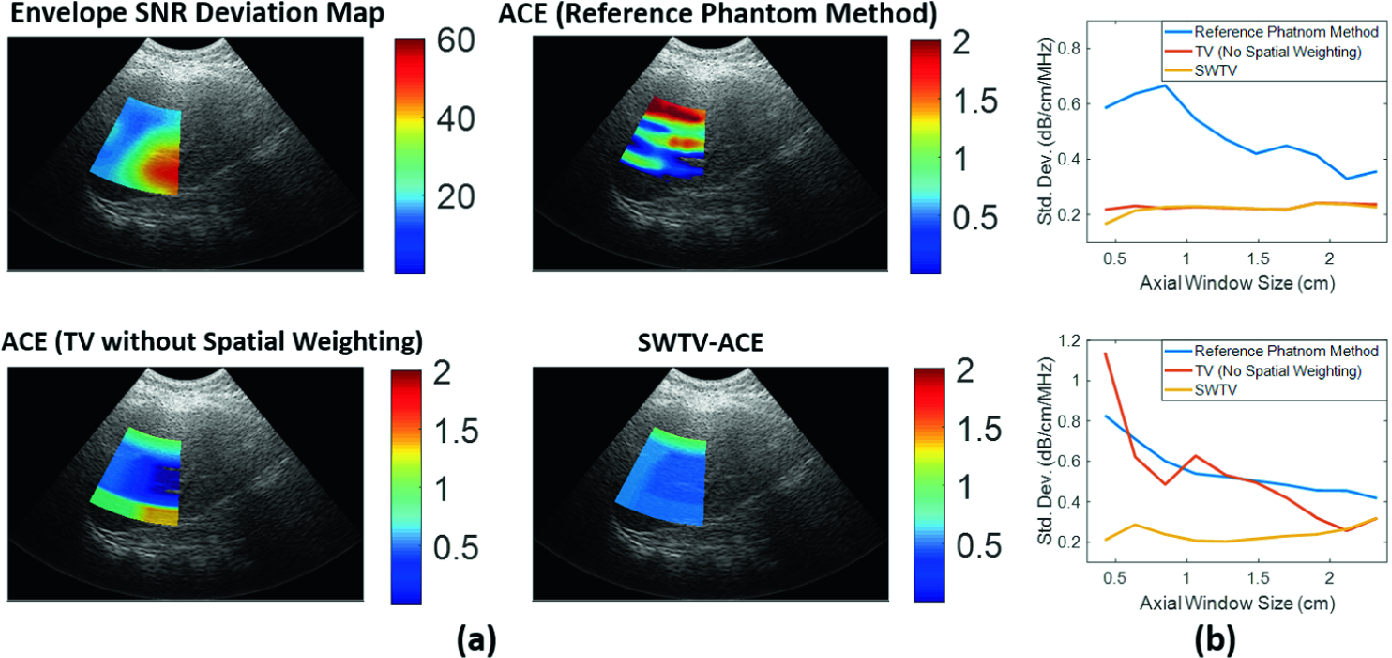
Figure 1: (a) An example of ACE computation for in vivo human liver using three different methods. (b) Effect of window size on estimation variance of ACE for a homogeneous region-of-interest (top) and inhomogeneous region-of-interest (bottom).
MRI proton density fat fraction (MRI-PDFF) computed from the MRI data was used as a gold standard for hepatic steatosis quantification, which strongly correlates
with histological steatosis grading.
The correlation between ACE computed using the reference phantom method to MRI-PDFF was 0.44 (p = 0.3838). The TV method yields a better correlation performance
(r = 0.71, p = 0.1132). The SWTV-ACE method outperforms both of these methods with a correlation of 0.953 (p =.003). Therefore, SWTV-ACE method demonstrated an
improved correlation with MRI-PDFF even with small window size, extending the trade-off between window size and precision inherent in conventional ACE
computation.
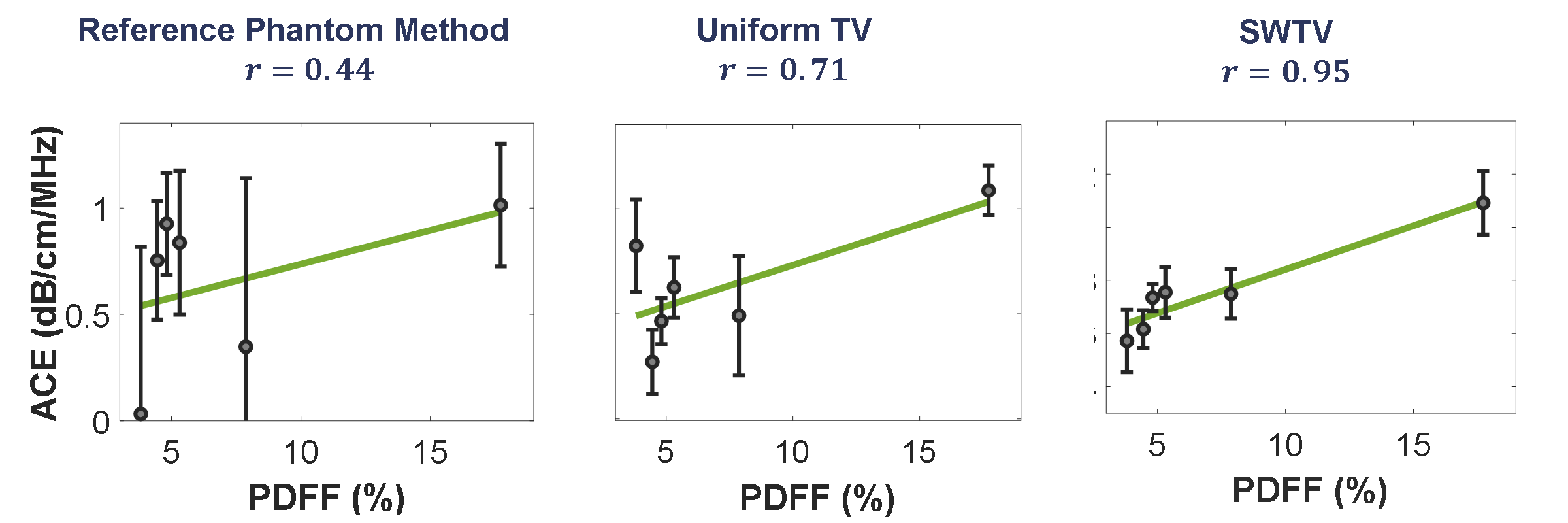
Figure 2: In vivo human liver ACE from six patients and their correlation with proton density fat fraction. The error bars show the standard deviation whereas the square represents the mean calculated over a region-of-interest.
The strong correlation of the ACE measurements with the current gold-standard MRI-PDFF demonstrated the potential application of the proposed method for the detection of liver steatosis.
1. Deeba, Farah, et al. "SWTV-ACE: spatially weighted regularization based attenuation coefficient estimation method for hepatic steatosis detection." International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer, Cham, 2019.
2. Deeba, Farah, et al. "3D ACE: Attenuation Coefficient Estimation in 3D for the Detection of Hepatic Steatosis." HEPATOLOGY. Vol. 68. 111 RIVER ST, HOBOKEN 07030-5774, NJ USA: WILEY, 2018.